The team’s projects consist in studying molecular and cellular mechanisms that allow cells (especially hepatic cells) to adapt to a variety of stresses like oxygen, nutrient or growth factor deprivation, oxidative stress, lipid accumulation or response to anticancer chemotherapy or to viral infections. This adaptation allows their survival in the case stresses are not too intense or prolonged. Alternatively, cells may die.
We are studying two facets of these phenomena with the downstream objective to identify new markers of stress and potential new therapeutic targets in cancer cells: i) how the adaptive mechanisms involved are spatially and temporally coordinated; and ii) how excessive adaptation might promote cell tolerance to death signals and unwanted survival of cells that would bear genome alterations, thereby participating in cancerogenesis.
We especially focus on studying four great molecular and cellular actors and their functional links:
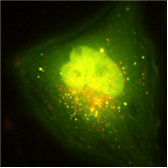 Autophagy
Autophagy
Autophagy (i.e. the self digestion) of cellular constituents allows cell survival to deprivation but also the elimination of damaged organelles like mitochondria or small parts of nuclei that concentrate damaged DNA. Autophagy is an inducible and plays a central role in adaptive cell survival and in specific forms of cell death. It can also be subverted from its physiological functions by pathogens like viruses.
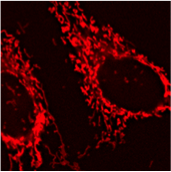
Mitochondria
Mitochondria are essential to trigger forms of cell death and participate in the induction of autophagy. We especially focus on mitochondrial dynamics which means their fission into smaller organelles or fusion in an extended network. This dynamics is key to control the fate of damaged mitochondrial material or the release reactive oxygen species that transmit early stress signals but may also alter proteins, lipids or genetic material.
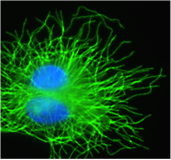
Mitrotubules
Microtubules, which belong to the cell’s skeleton. Their dynamics and plasticity have a major function in the spatio-temporal coordination of autophagy, mitochondria dynamics, and of several intracellular signals involved in stress adaptation. We especially study how molecules that constitute microtubules are modified during stress and how these modifications are linked to autophagy and mitochondria dynamics. We also analyse how such modifications participate in cell resistance to certain anticancer chemotherapies and in the cycle of hepatitis viruses.
Cellular signalling
Cellular signalling pathways, some of which are activated or silenced in stress response and which participate in sustaining this response. These mechanisms involve the propagation of danger signals or of stress markers inside and outside cells, the control of autophagy induction or that of mitochondria dynamics and fate. Some of these pathways are organized by microtubules and require their dynamic behaviour to function while others act indirectly on adaptation by controlling microtubule dynamics.